See if you Qualify
Take our 1-minute quiz.
Take our 1-minute quiz.
Although many are under the impression that the Food and Drug Administration (FDA) requires medical device manufacturers to undergo rigorous tests prior to obtaining approval for their product, this is not the case for the hernia mesh device.
In fact, the FDA allowed hernia mesh manufacturers to use the 510(k) clearance process, which is a fast-track program that can rush products onto the market. Unfortunately, this process allowed the sale of thousands of defective hernia mesh products which led to severe complications in many patients that even required revision surgery to fix.
On this page, we explain everything about the hernia mesh medical device, from common complications to which companies are under fire to trial dates for lawsuits in 2023 and beyond. This information is provided to help you answer the question you might be asking yourself or you might possibly ask a hernia mesh attorney in the future: do I qualify for compensation?
November 8, 2023 – Day 14 of Stinson Trial: Day 14 of the Stinson bellwether hernia mesh trial saw testimony from defense witnesses Dr. Stephen Badylak and Maureen Reitman. A motion was filed for a unified damages verdict form to minimize juror confusion and prevent appeals.
November 3, 2023 – Stinson Trial Continues: Bard called Mr. Stinson’s surgeon, a pathology expert, and a retired Davol marketing director to testify.
November 1, 2023 – Defense Expert Testifies: The entire day revolved around Dr. Pomerants’ testimony, including direct, cross, and redirect examination.
October 31, 2023 – Plaintiff’s Rests in Stinson: The plaintiff rested his case, and the defense called Dr. Boris Pomerants to begin their case. Dr. Pomerants presented alternative causes for the plaintiff’s injuries.
October 26, 2023 – Stinton v. Bard Enters the Ninth Day: Significant testimonies were heard from the plaintiff and a key expert witness linking the defective mesh to Mr. Stinson’s injuries.
October 17, 2023 – MDL Posts First Ever Monthly Decrease: The Bard hernia mesh MDL saw its first monthly decrease in pending cases, indicating a potential slowdown or the start of settlements.
October 10, 2023 – Facts of Bellwether Trial: Details of Stinson v. Davol Inc., et al. were summarized, highlighting the complications and pain experienced by Stinson post-surgery.
October 2, 2023 – Stinson on Deck: Expectations were set for the Stinson trial settlement, with Bard seen as likely to settle.
September 23, 2023 – Coviden MDL Has Trial Dates in 2025: Trial dates for six bellwether cases in the Covidien hernia mesh MDL were set for early 2025, with implications for both Covidien and Bard MDLs.
September 18, 2023 – MDL Surpasses 20,000 Cases: The Bard hernia mesh MDL added around 700 new cases in three months, reaching 20,405 cases.
September 1, 2023 – Bellwether Trials Around the Corner: The next bellwether test trial in the hernia mesh class action against C.R. Bard was confirmed for October 16, 2023.
August 16, 2023 – 20,126 Bard Hernia Mesh Lawsuits: The Bard MDL continued to grow, adding nearly 200 new lawsuits in a month.
August 2, 2023 – Bellwether Trial Set for October: The third bellwether trial in the Bard hernia mesh MDL was scheduled for October 16, 2023.
July 27, 2023 – Endless Litigation: A plaintiff’s death was reported, highlighting the prolonged nature of the MDL.
July 18, 2023 – Over 200 New Cases Added to MDL: An influx of 228 new cases was seen in the Bard hernia mesh MDL.
July 15, 2023 – FDA Offers Guidance: The FDA released a guide on the risks and benefits of hernia surgery mesh.
July 1, 2023 – Post-Trial Conversations with Jurors: Following a defense verdict in the first bellwether trial, discussions about juror feedback led to allegations of ethics breaches, later resolved by the Court.
June 23, 2023 – MDL Trials Stay on Track: Judge Sargus denied a defense request to replace cases in future bellwether trials.
June 16, 2023 – MDL Continues to Grow: The Bard hernia mesh MDL saw an addition of 231 new plaintiffs.
June 13, 2023 – Judge Excludes Evidence: Evidence unrelated to Stinson’s injury was excluded from the trial.
June 8, 2023 – Covidien Science Day: A Science Day was scheduled in the Covidien hernia mesh MDL.
June 1, 2023 – Hernia Mesh Settlements in Georgia: Johnson & Johnson and Ethicon Inc. settled 161 hernia mesh cases.
April 22, 2023 – New Bellwether Date in October: The third bellwether trial in the Bard hernia mesh MDL was rescheduled for October 16, 2023.
March 20, 2023 – Bellwether Trials Update: The third bellwether trial in the Bard hernia mesh MDL was postponed.
February 9, 2023 – Bellwether Trials Update: The Stinson case trial was scheduled to start on May 15, 2023.
January 26, 2023 – Bard MDL Update: An overview of the status of hernia mesh lawsuits was provided.
January 18, 2023 – Bard MDL Update: 176 new cases were added to the Bard hernia mesh MDL.
January 3, 2022 – Covidien MDL Update: The Covidien hernia mesh MDL started with a focus on coordinating discovery between state and federal actions.
Hernias occur when there is weakness in the muscular abdominal wall, which consequently allows tissue or part of the intestine to poke through the weak area.
Doctors started using the hernia mesh medical device because of the idea that it would lower the chances of a hernia coming back, a condition known as hernia recurrence.
Unfortunately, due to fast-tracking surgical hernia mesh implants, we still cannot call hernia recurrence a thing of the past. Many of these implants have also been known to fail due to design defects, leading to severe complications that ultimately resulted in a revision surgery for countless patients.
Many hernia mesh patients who have undergone surgery to fix their hernias with the use of surgical hernia mesh implants have also reported numerous side effects.
The use of a can result in a plethora of injuries, including:
The majority of surgeries make use of . However, the polypropylene mesh, a kind of , has been known to cause more problems for patients, including allergies and mesh rejection.
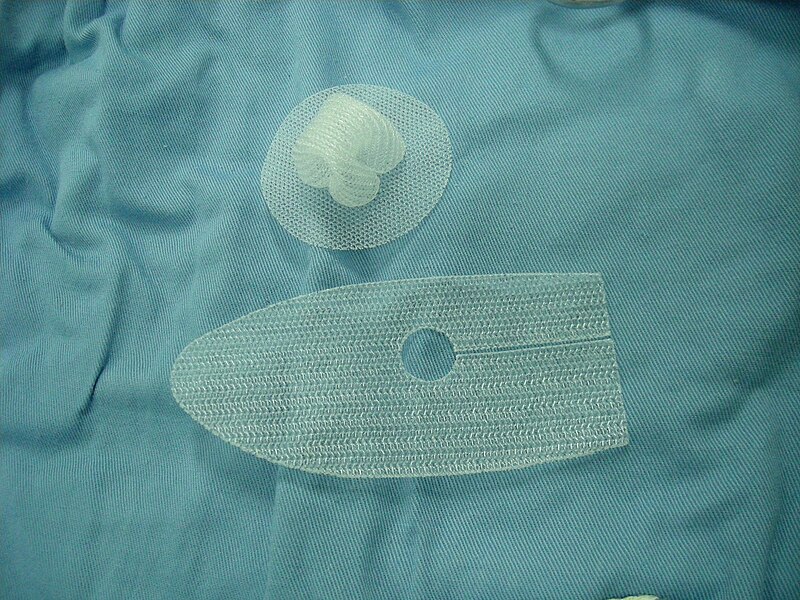
The first hernia mesh recall was issued in late 2005 by Davol Inc., a subsidiary of C.R. Bard. The recall was extended in 2006 and again in 2007.
In 2014, the Food & Drug Administration announced a number of hernia mesh recalls with reasons ranging from poor performance to packaging errors to adverse events. Companies targeted by the FDA included C.R. Bard, Ethicon Inc., and Atrium Medical Corporation.
Ethicon Inc. recalled its Physiomesh product in the United States in May 2016 after the implant was linked to several cases of hernia recurrence and revision surgery. Parent company Johnson and Johnson has also recalled Physiomesh in Europe and Australia due to complications.
Like most hernia mesh implants, Physiomesh is made from polypropylene. This is the same material used in bladder slings and transvaginal mesh. Both of which have resulted in thousands of lawsuits.
To date, there have been several lawsuits filed involving defective hernia meshes, including a suit filed in September 2016 by a Florida woman, Joann Quinn, who alleged that she suffered bowel adhesions that required surgery due to Physiomesh. Now that the doctor reported that removal of all the adhesions is no longer possible, she could suffer from permanent complications.
Still, Ethicon Inc. has stated that it has no idea why the Physiomesh is defective nor was it aware of any way to limit complications for those patients who already had the defective mesh implanted for hernia repair surgery. You can start a hernia mesh lawsuit today by contacting us and we will help you find the right hernia mesh lawyer.
Parietex and Parietex Composite Mesh
Covidien, now known as Medtronic Minimally Invasive Therapies, first used polyester instead of polypropylene in a . The company’s Parietex was brought to the market in 1999. The results were not good. Many subjects experienced bowel issues, adhesions, pain, and infections as a result of this particular hernia mesh.
Eventually, the company moved on to the Parietex Composite Mesh, which was made of polyester with a collagen barrier. The idea was that the collagen barrier would prevent the polyester base from adhering the bowels. Unfortunately, the barrier failed to work, and many patients continued to report bowel issues.
Next, Covidien developed the Parietex ProGrip Mesh System: a “self-fixating” mesh system used in hernia procedures, which featured thousands of hooks designed to keep the mesh in place. However, the hooks caused victims considerable pain and injuries, and made it more difficult for doctors to remove the mesh.
Studies show that companies that manufacture mesh products make around $100,000,000.00 a year. It does not appear that the thousands of victims whose lives have been ruined by the hernia mesh are getting in the way of this lucrative business.
One of the first hernia mesh implants to be recalled was C.R. Bard’s Kugel hernia mesh patches. It was approved in the 1990s and has been implanted over a million times.
The implant was made of polypropylene and contained a ring around the mesh. In some cases, this ring would break. Once this happens, the polypropylene would shrink to a size that was smaller than the size of the ring, exposing the mesh and and causing it to break and perforate the bowels or other organs.
Hernia mesh manufacturer Davol Inc., a subsidiary of C.R. Bard, recalled the Kugel meshes in 2005, 2006, and 2007. However, lawsuits are still pending against the surgical mesh, including a lawsuit in Rhode Island filed by Wayne Smith, who had it implanted in 2005, prior to the first recall. Shortly after his surgery, he began to complain of abdominal pain and tenderness around the surgical site and it was recommended for him to undergo mesh removal.
C.R. Bard and Davol Inc. attempted to have the lawsuit dismissed, claiming that the plaintiff’s claims did not satisfy the laws of Rhode Island. However, the judge presiding over the case has refused to dismiss the case.
C.R. Bard manufactures several other hernia mesh implants which have been named in lawsuits due to complications caused by their design defects. These are the PerFix Plug, 3DMax, and Ventralex ST mesh implants. Make sure you check this page often to keep up with the C.R. Bard hernia mesh lawsuits.
One way to alert the regulator of injuries is to ask an attorney to file a hernia mesh lawsuit against the manufacturer of the allegedly defective product. Generally, the more lawsuits that are filed, the more likely it is that the agency will take notice of the complications related to the medical device.
If the defective hernia mesh implant fails within three years after surgery, and the same surgeon who implanted the mesh also removes it, that surgeon must report that injury to the manufacturer. If surgeons continue to file these reports to manufacturers, those companies are legally obligated to inform the U.S. regulatory agency about the situation.
Although the FDA has posted a statement on its website regarding hernia meshes and the possible complications involved, the American regulatory agency seems resistant to admitting that several approved hernia mesh products are defective.
The statement notes that the most serious reports that involved hernia mesh products were later recalled – a claim most attorneys would dispute.
Moreover, after sending a warning to Atrium Medical Corporation in 2012 regarding complaints against their mesh product and eventually putting a stop to the device’s production, the FDA has not targeted any other hernia mesh manufacturer after that.
This, despite plenty of research linking defective meshes to serious injuries, complications, and additional surgery.
Below are several, recent hernia mesh studies and a summary of their results.
June 2017 – Study on Deterioration of Polypropylene Hernia Repair Meshes
The study attempted to investigate how hernia meshes can change within the body. The “oxidative stress at the site of implantation” can sometimes cause the implants to lose their structural integrity. This can result in stiffening or even shrinkage of the mesh, often causing chronic pain in patients.
August 2016 – Two-Year Study on Patients Who Received a Hernia Mesh
Over 600 subjects were studied for two years after they were implanted with a mesh for hernia repair surgery. Around 31 percent of those patients suffered some type of complication within two years. These side effects included tissue necrosis, fistula, dehiscence, cellulitis, and other types of physical injury.
Patients who had a preoperative MRSA+ infection on any site are at an even greater risk of developing complications.
August 2015 – Study Indicating the Impact of MRSA+ Infection on Likelihood of Hernia Mesh Infections
768 patients went through a hernia repair procedure which included the surgical implantation of hernia mesh. Around 10 percent ended up with a hernia mesh tissue infection, while 33 percent of the subjects who had a preoperative MRSA+ infection suffered from a hernia mesh infection.
1. Medical News Today: Types and treatments for hernia
2. WebMD: Understanding Hernia Basics
3. FDA.gov: Hernia Surgical Mesh Implants
4. BBC: Hernia mesh complications ‘affect more than 100,000’
5. Gutresolution: Hernia Mesh – A Story of Deception, Tragedy and Hope
6. FDA.gov: Recalls, Corrections and Removals (Devices)
7. Massdevice: J&J’s Ethicon recalls Physiomesh flexible composite hernia mesh
8. Ethicon: ETHICON PHYSIOMESH™ Open Flexible Composite Mesh Device
10. Pacermonitor: Quinn v. Ethicon, Inc. et al
11. Masstortnexus: Documents – Physiomesh Complaint Huff vs Ethicon
12. Pubmed: In vitro study on the deterioration of polypropylene hernia repair meshes.
Page Contents
November 2021 update: The judge set the next trial to be on January 10, 2022. Hopefully, the upcoming trial will result in a favorable verdict for the plaintiffs.
October 2021 update: To avoid the possibility of a second bellwether trial, the defense filed a motion for summary judgment. However, the presiding judge denied the defendant’s motion.
September 2021 update: The first bellwether trial resulted in a loss for the plaintiff, Steven Johns. The case was a defense pick. C.R. Bard scored a surprising victory in the first test trial. The jury found in favor of the defendants in all counts and awarded no damages to Mr. Johns.
Overall, trials during the COVID pandemic are not easy for like Johns. Plaintiffs’ attorneys had to push these cases to trial for the welfare of all victims.
To date, over 20,000 lawsuits are pending against mesh manufacturers Atrium Medical Corp., Ethicon, and Davol Inc./C.R. Bard. Hundreds more are also pending in state courts across the country.
January 2021 update: According to the Judicial Panel on , there are a total of over 70,000 cases pending, including those filed against C.R. Bard, Inc., Boston Scientific Corp., and Ethicon Inc.
Meanwhile, class action against Atrium Medical Corporation has continued to gain traction over the last 6 months, with an average of 39 cases filed each month, compared to an average of just 24 in the previous months.
January 2020 update: As of January 2020, more than 2,000 pending lawsuits have been centralized in the MDL.
The mass tort against Ethicon (MDL -2782 IN RE: Ethicon Physiomesh Flexible Composite Mesh Products Liability Litigation) was set for trial in June 2020 by Honorable Judge Story.
With dozens of pending, it is possible that class action lawsuits could be filed, although none have been filed as of yet. We constantly update this post whenever new information is readily available. Please don’t hesitate to fill out the form located at the top of this page for a free case evaluation. A lawyer will be ready to help you.
As of this writing, no has been reached in any of the active against Atrium, Bard, and Ethicon. Therefore, we don’t know exactly how much these cases will be worth.
However, in the past, there were instances where mesh manufacturers were ordered to pay compensation to plaintiffs.
For instance, in April 2017, Ethicon Inc. was ordered to pay a total of approximately $20 million in damages by a Philadelphia Jury. The jury concluded that a TVT-Secur mesh implanted in a New Jersey woman was defectively designed and caused her serious injuries.
The following year, Atrium Medical Corporation was forced to pay $11 million to resolve around 3,000 .
To date, the largest amount is $184 million paid by C.R. Bard to resolve around 3,000 cases in 2011.
For now, however, because each case is unique, it’s hard to give an estimate on the settlement value of these lawsuits. There are, however, some factors that may influence a settlement, including:
Drugwatcher helps you to stand up against producers of dangerous drugs and medical devices.